Snake_Baker
The one true King of the North
- Apr 24, 2013
- 81,024
- 153,169
- AFL Club
- North Melbourne
- Other Teams
- Essendon Lawn Bowls Club
- Banned
- #1
Are there any organic chem folks out there that can answer a ring structure question for me?
I am finalising a paper on the grignard synthesis of triphenylmethanol and would like some tips on proper carbon numbering of the compound:

I am trying to identify the proper nomenclature for the carbon molecule intersecting the OH group and benzene rings.
Would this be identified as a tertiary alcohol carbon or a phenyl carbon?
I am finalising a paper on the grignard synthesis of triphenylmethanol and would like some tips on proper carbon numbering of the compound:
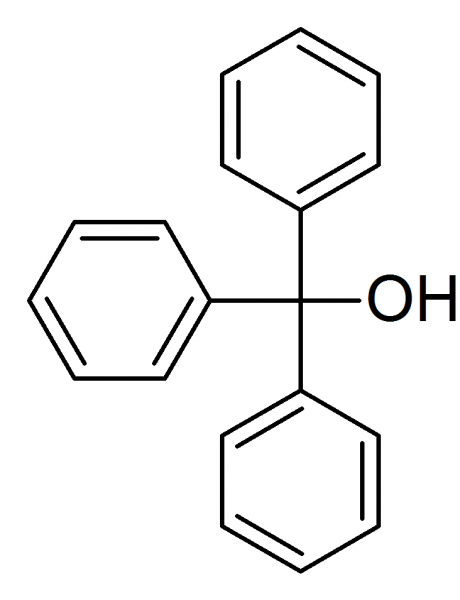
I am trying to identify the proper nomenclature for the carbon molecule intersecting the OH group and benzene rings.
Would this be identified as a tertiary alcohol carbon or a phenyl carbon?




