Snake_Baker
The one true King of the North
- Apr 24, 2013
- 81,024
- 153,198
- AFL Club
- North Melbourne
- Other Teams
- Essendon Lawn Bowls Club
- Banned
- #1
Another piece in the puzzle:
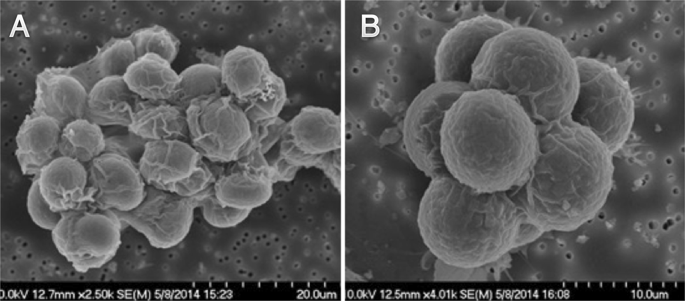
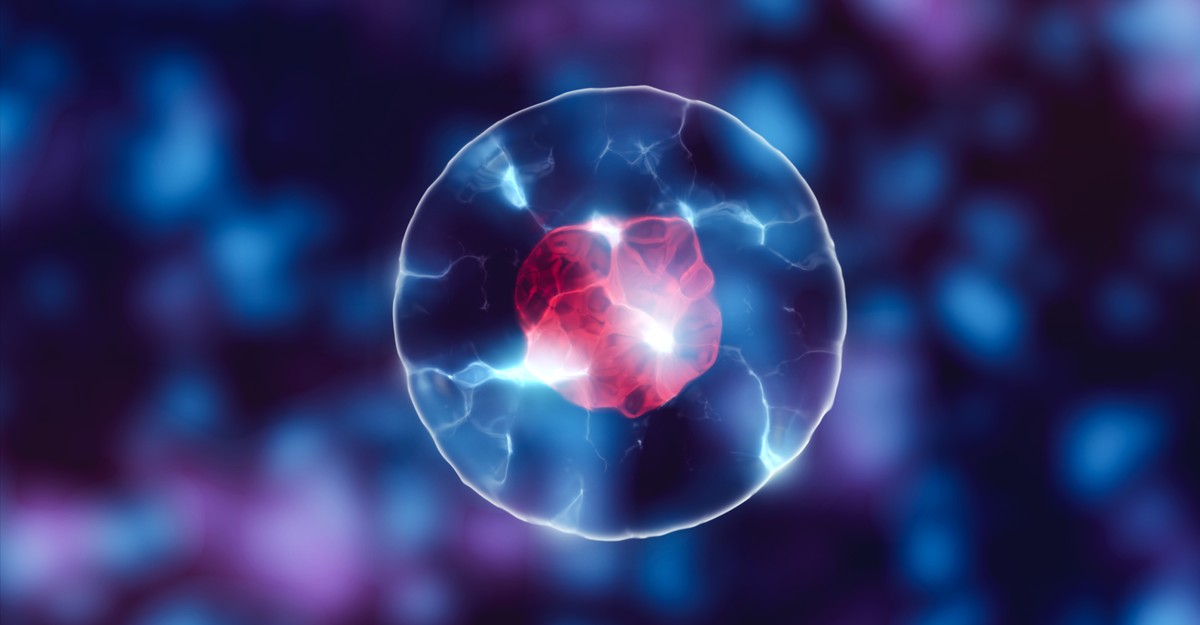
When Caitlin Cornell looked down her microscope, she saw large bright spots against a black background. They resembled miniature suns, blazing against the backdrop of space. And when Cornell showed the spots to her supervisor, Sarah Keller, a chemist at the University of Washington, “we got really excited,” she recalls. “It was a bit of an ‘Aha!’ moment.” Those spots, she realized, might help address a long-standing puzzle about the origin of life itself.
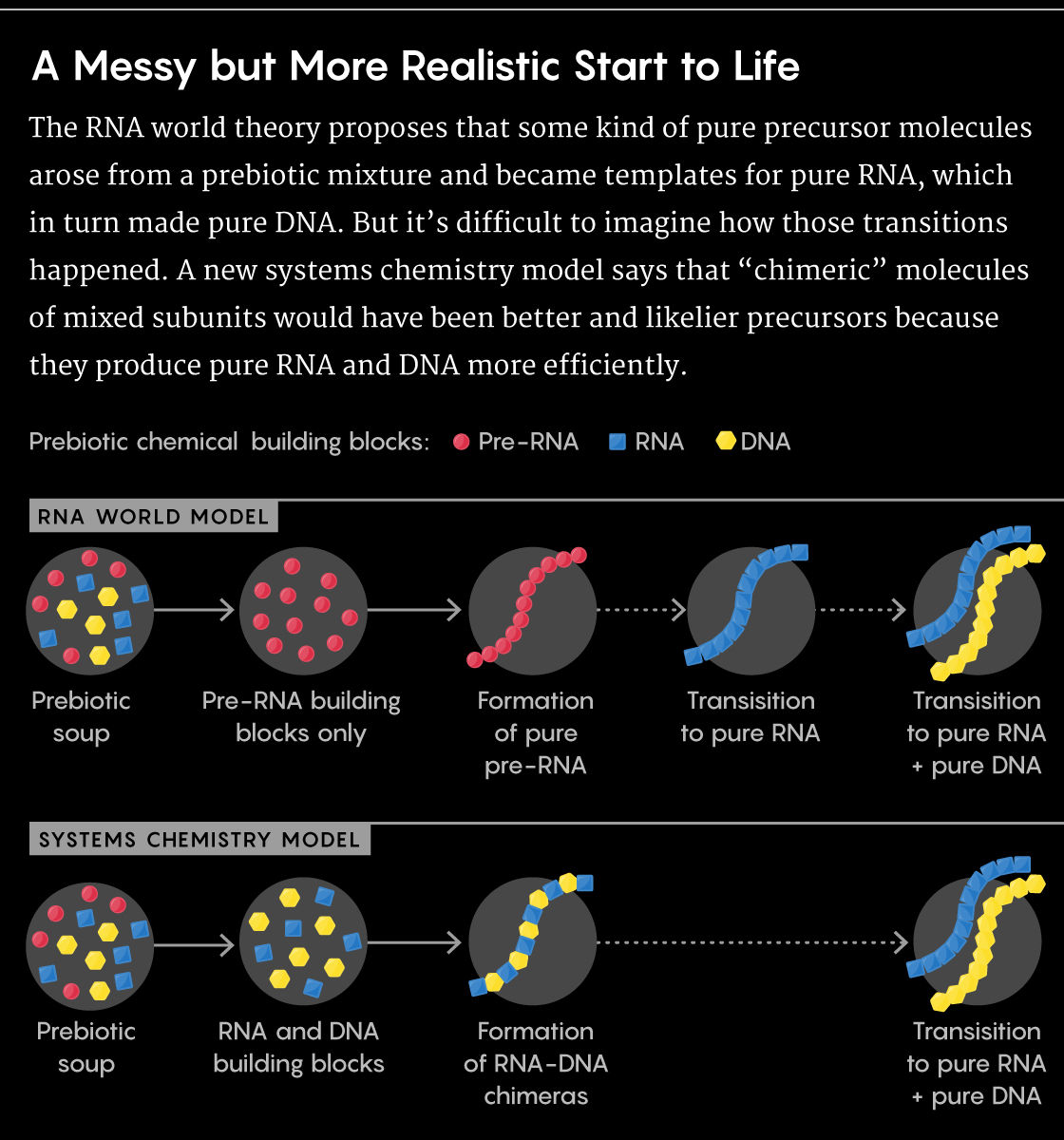
The cells that make up all living things, despite their endless variations, contain three fundamental elements. There are molecules that encode information and can be copied—DNA and its simpler relative, RNA. There are proteins—workhorse molecules that perform important tasks. And encapsulating them all, there’s a membrane made from fatty acids. Go back far enough in time, before animals and plants and even bacteria existed, and you’d find that the precursor of all life—what scientists call a “protocell”—likely had this same trinity of parts: RNA and proteins, in a membrane. As the physicist Freeman Dyson once said, “Life began with little bags of garbage.”
The bags—the membranes—were crucial. Without something to corral the other molecules, they would all just float away, diffusing into the world and achieving nothing. By concentrating them, membranes transformed an inanimate world of disordered chemicals into one teeming with redwoods and redstarts, elephants and E. coli, humans and hagfish. Life, at its core, is about creating compartments. And that’s much easier and much harder than it might seem.
First, the easy bit. Early cell membranes were built from fatty acids—molecules that look like lollipops, with round heads and long tails. The heads enjoy the company of water; the tails despise it. So, when placed in water, fatty acids self-assemble into hollow spheres, with the water-hating tails pointing inward and the water-loving heads on the surface. These spheres can enclose RNA and proteins, making protocells. Fatty acids, then, can automatically create the compartments that were necessary for life to emerge. It almost seems too good to be true.
And it is, for two reasons. Life first arose in salty oceans, and salt catastrophically destabilizes the fatty-acid spheres. Also, certain ions, including magnesium and iron, cause the spheres to collapse, which is problematic since RNA—another key component of early protocells—requires these ions. How, then, could life possibly have arisen, when the compartments it needs are destroyed by the conditions in which it first emerged, and by the very ingredients it needs to thrive?
Caitlin Cornell and Sarah Keller have an answer to this paradox. They’ve shown that the spheres can withstand both salt and magnesium ions, as long as they’re in the presence of amino acids—the simple molecules that are the building blocks of proteins. The little suns that Cornell saw under her microscope were mixtures of amino acids and fatty acids, holding their spherical shape in the presence of salt.
I find that utterly magical. It means that two of the essential components of life, a protocell’s membrane and its proteins, provided the conditions for each other to exist. By sticking to the fatty acids, the amino acids gave them stability. In turn, the fatty acids concentrated the amino acids, perhaps encouraging them to coalesce into proteins. From the very beginning, these partners were locked in a two-step dance that continued for 3.5 billion years, and helped create all the richness of biology from a starting place of mere chemistry. “I agree completely,” Keller tells me. “It’s completely magical. You need those two parts together.”
“It’s fantastic work,” says Neal Devaraj, of UC San Diego. “Their suggestion that membranes could promote the synthesis of [proteins] is really fascinating.”
 www.theatlantic.com
www.theatlantic.com
When Caitlin Cornell looked down her microscope, she saw large bright spots against a black background. They resembled miniature suns, blazing against the backdrop of space. And when Cornell showed the spots to her supervisor, Sarah Keller, a chemist at the University of Washington, “we got really excited,” she recalls. “It was a bit of an ‘Aha!’ moment.” Those spots, she realized, might help address a long-standing puzzle about the origin of life itself.
The cells that make up all living things, despite their endless variations, contain three fundamental elements. There are molecules that encode information and can be copied—DNA and its simpler relative, RNA. There are proteins—workhorse molecules that perform important tasks. And encapsulating them all, there’s a membrane made from fatty acids. Go back far enough in time, before animals and plants and even bacteria existed, and you’d find that the precursor of all life—what scientists call a “protocell”—likely had this same trinity of parts: RNA and proteins, in a membrane. As the physicist Freeman Dyson once said, “Life began with little bags of garbage.”
The bags—the membranes—were crucial. Without something to corral the other molecules, they would all just float away, diffusing into the world and achieving nothing. By concentrating them, membranes transformed an inanimate world of disordered chemicals into one teeming with redwoods and redstarts, elephants and E. coli, humans and hagfish. Life, at its core, is about creating compartments. And that’s much easier and much harder than it might seem.
First, the easy bit. Early cell membranes were built from fatty acids—molecules that look like lollipops, with round heads and long tails. The heads enjoy the company of water; the tails despise it. So, when placed in water, fatty acids self-assemble into hollow spheres, with the water-hating tails pointing inward and the water-loving heads on the surface. These spheres can enclose RNA and proteins, making protocells. Fatty acids, then, can automatically create the compartments that were necessary for life to emerge. It almost seems too good to be true.
And it is, for two reasons. Life first arose in salty oceans, and salt catastrophically destabilizes the fatty-acid spheres. Also, certain ions, including magnesium and iron, cause the spheres to collapse, which is problematic since RNA—another key component of early protocells—requires these ions. How, then, could life possibly have arisen, when the compartments it needs are destroyed by the conditions in which it first emerged, and by the very ingredients it needs to thrive?
Caitlin Cornell and Sarah Keller have an answer to this paradox. They’ve shown that the spheres can withstand both salt and magnesium ions, as long as they’re in the presence of amino acids—the simple molecules that are the building blocks of proteins. The little suns that Cornell saw under her microscope were mixtures of amino acids and fatty acids, holding their spherical shape in the presence of salt.
I find that utterly magical. It means that two of the essential components of life, a protocell’s membrane and its proteins, provided the conditions for each other to exist. By sticking to the fatty acids, the amino acids gave them stability. In turn, the fatty acids concentrated the amino acids, perhaps encouraging them to coalesce into proteins. From the very beginning, these partners were locked in a two-step dance that continued for 3.5 billion years, and helped create all the richness of biology from a starting place of mere chemistry. “I agree completely,” Keller tells me. “It’s completely magical. You need those two parts together.”
“It’s fantastic work,” says Neal Devaraj, of UC San Diego. “Their suggestion that membranes could promote the synthesis of [proteins] is really fascinating.”

A New Clue to How Life Originated
A long-standing mystery about early cells has a solution—and it’s a rather magical one.